Connect with a Global Community of 80+ mRNA Experts
As mRNA therapeutics evolve beyond vaccines and advance into different disease indications, novel modalities and advanced delivery systems, the industry faces mounting pressure to maintain process and CMC excellence. Biopharma leaders are actively seeking partners who can help them with reliable, high quality raw materials, manufacturing capabilities, GMP scale, purification, formulation and fill-and-finish services that withstand global regulatory scrutiny.
The demand for cutting-edge technologies and services that strengthen process reproducibility, reliability and control has never been greater.
The 4th mRNA Process Development & CMC Summit is Europe’s only dedicated forum uniting process, CMC, manufacturing, quality, analytical and regulatory decision-makers across pharma, biotech, and academia. Partnering with us provides a powerful platform to:
- Position your organisation at the forefront of process innovation and CMC excellence in mRNA therapeutics and vaccines.
- Showcase your solutions to optimise accuracy, reproducibility, and compliance across all mRNA production functions, to build robust regulatory packages
- Connect directly with senior Process, CMC, Analytical and Quality leaders from top biopharma actively seeking solutions and driving transformation in mRNA production, to form with genuine connections.
By joining with this summit, you’ll not only gain visibility among the industry’s most influential stakeholders but also play a central role in defining the process development gold standards for next-generation mRNA therapeutics and vaccines.

What to Expect?
Raise Your Brand Awareness
Position your organisation at the forefront of mRNA process innovation to ensure your brand is seen by senior decision-makers from global pharma, biotech, and regulatory bodies actively shaping the standards for mRNA therapeutics and vaccines.
Uncover the Next Market Trend
Gain firsthand insights into emerging manufacturing, process, quality control and regulatory priorities, that are driving the next generation of mRNA therapeutics and vaccines. Stay ahead of competitors with intelligence from global innovators and regulators defining the future of mRNA.
Distinguish Yourself from the Crowd
Learn from our unrivalled market intelligence of where key pain points lie for potential clients and tailor your presentations in line with their needs. Demonstrate real-world value through thought-leadership sessions, case studies, and live discussions with key stakeholders.
Brush Shoulders with Industry Experts
Network with leading scientists, directors, heads, CXOs and regulatory authorities in an interactive, discussion-driven setting. Exchange ideas, benchmark strategies, and establish meaningful collaborations with the experts setting the agenda for mRNA process development and CMC worldwide.
Generate Commercial Opportunities
Engage directly with prospective partners, collaborators, and clients actively seeking solutions to their process and CMC challenges. From early discovery to GMP readiness, build new business relationships and accelerate your commercial pipeline through qualified, face-to-face connections.
Key Services & Solutions
Our expert attendees from mRNA biopharma are looking for service and solution providers with capabilities in the below areas but not limited to:
CDMO & Manufacturing Enablement
End-to-end development and manufacturing capabilities that support mRNA programmes from early process development through GMP production. From tech transfer and scale-up to clinical and commercial supply, enable robust, compliant manufacturing strategies that reduce risk, timelines, and operational complexity across modalities.
DNA & IVT Raw Material Solutions
Advanced platforms for plasmid DNA, enzymes, caps and critical reagents that underpin high-quality mRNA production. Addressing challenges in scalability, purity, turnaround time and regulatory scrutiny, these solutions strengthen upstream process reliability and CMC readiness from the very first step.
Bioprocessing Technologies & Scale Infrastructure
Innovative equipment, consumables and process technologies that enable efficient, reproducible mRNA production at scale. From IVT reaction control to purification and concentration, optimise yields, manage impurities and support flexible manufacturing across development stages.
Digitalisation & Process Control Strategies
Integrated automation, monitoring and data-driven tools that enhance process understanding, control and reproducibility across mRNA manufacturing workflows. Enable smarter scale-up, faster troubleshooting and stronger alignment with evolving regulatory expectations for process robustness and lifecycle management.
Hear What Our Past Sponsors Have to Say

The size and focus of the meeting made this conference super valuable
Sabrina Baffert, Director Molecular Biology, Primrose Bio

It was a beautiful event, good people, good atmosphere, nice conversations
Dirk Forberger, Chief Executive Officer, RoweMed AG - Medical 4 Li
Audience Composition
Company Type
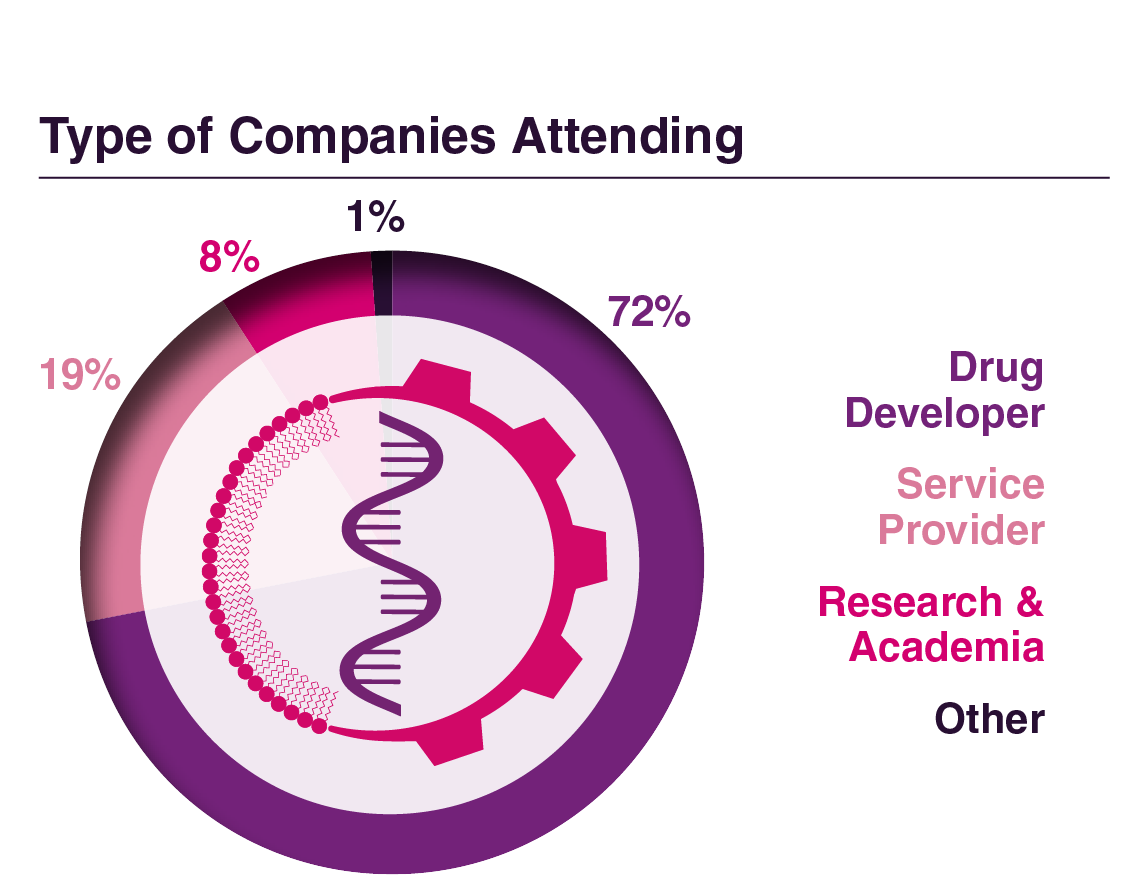
Attendee Seniority
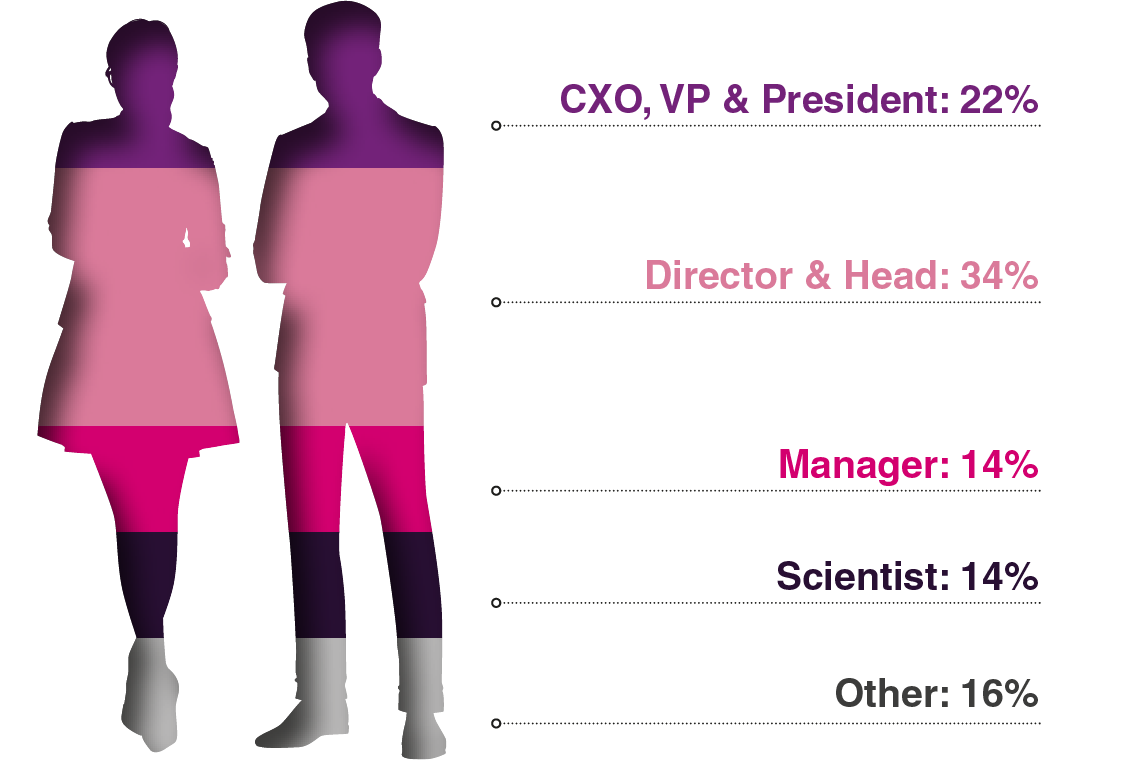
Attending Companies Include



Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.

William Hobson-Corbett
Senior Partnerships Director



